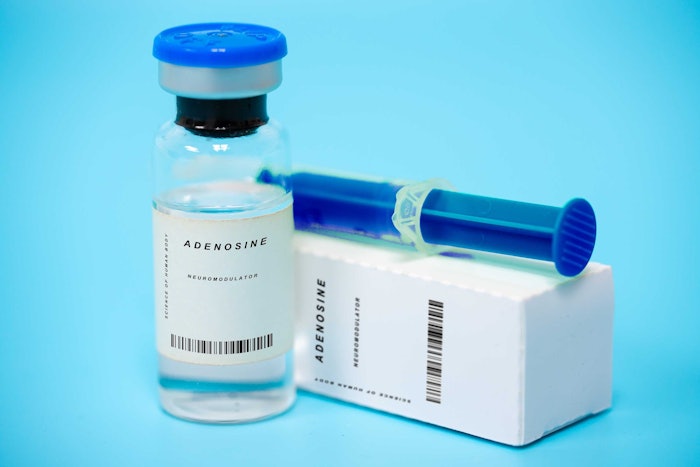
The American Academy of Facial Plastic and Reconstructive Surgery (AAFPRS) is urging consumers to remain vigilant as counterfeit and unapproved neuromodulators continue to enter the market.
In a deeply globalized market like the one we have today, it's all too easy for all sorts of products and precursors to cross borders, making it difficult to keep up from a regulatory standpoint.
Following recent FDA warning letters issued to more than a dozen companies across the U.S. and internationally for selling unauthorized versions of botulinum toxin type A—the neurotoxin used to temporarily relax facial muscles and smooth wrinkles— the AAFPRS warns that these counterfeit products pose serious health risks.
The Urgency
“As counterfeit injectable products continue to surface, it’s imperative for patients to prioritize their safety,” says Anthony E. Brissett, MD, FACS, president of the AAFPRS. “Anyone considering cosmetic treatments should take care to seek out medically trained professionals who use only properly handled, FDA-approved medications."
This matters because:
- Counterfeit products may be contaminated, incorrectly formulated or ineffective, exposing patients to dangerous chemicals and unpredictable effects.
- Even when the product is legitimate, injections performed by individuals without proper medical training can lead to irreversible complications, including nerve damage, drooping eyelids or facial muscles, severe asymmetry, breathing difficulties and blindness.
- At-home Do-It-Yourself (DIY) injections are especially hazardous, even with “real” product.
How to Advise Clients
- Clients should always verify the credentials of their treatment provider. If you have a network of trusted plastic surgeons, dermatologists or other qualified practitioners, a referral can be a good way to make this process easier for a client interested in getting a procedure.
- Clients should request to see the medication vial and its packaging. Show clients how they can identify proper labeling from counterfeits, show them where they can check the expiration dates and how to tell if a medication is from a licensed pharmaceutical company.
- Warn clients against purchasing neuromodulators for at-home use.
The AAFPRS recommends:
- Individuals seek injectable care from a licensed, board-certified physician trained in facial anatomy and injectables, specializing in plastic surgery of the face, head and neck, or under the direct supervision of such a physician.
- Report suspicious injectable products or unlicensed providers to your state medical board, and the FDA (via the FDA MedWatch Online Voluntary Reporting Form).
- Seek medical attention right away for any unusual symptoms after an injection.
Source: The American Academy of Facial Plastic and Reconstructive Surgery. (2025, December 10). Consumer Alert on the Rise of Counterfeit Neuromodulators [Press release]. https://www.aafprs.org/Media/Press_Releases/Consumer_Alert_on_the_Rise_of_Counterfeit_Neuromodulators.aspx