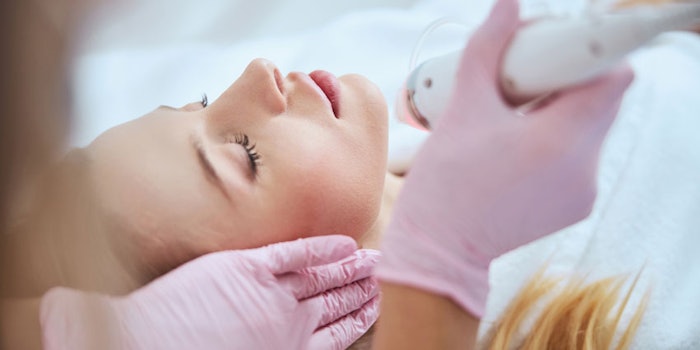
On September 20, 2021, the U.S. Food and Drug Administration issued the following consumer update regarding the safety and regulatory status of microneedling devices:
Being pricked by tiny needles may sound like a strange way to make your skin look better, but that’s the concept behind a skin care technique called microneedling. People are using microneedling in a variety of ways from reducing scarring, to treating fine lines and wrinkles. It is important to be aware of the risks as well as benefits associated with microneedling devices.
If you are thinking about having a microneedling procedure, the U.S. Food and Drug Administration recommends you choose a health care provider who is specially trained in microneedling. Talk with the provider to determine if you are a good candidate for microneedling—not everyone is. Discuss the benefits and risks, including the risk of infection if the microneedling tools are not cleaned or used properly.
What are microneedling products and how are they used?
While there are several types of microneedling products, they all have a key feature—they contain lots of small, thin needles. The needles may be:
- Fitted onto a cylinder and rolled across the skin (known as a dermal roller)
- Attached to a flat surface and stamped into the skin
- Arranged in a pattern on the tip of a pen-shaped instrument
Some products have needles that are so short they only touch the topmost layer of the skin, which is mainly dry, dead skin. Other products have longer needles that penetrate the skin and reach deeper layers that have living cells, nerves, and blood vessels.
The products may be manual, meaning they are operated by hand, or they may have a motor that moves the needles.
Are all microneedling products considered medical devices that are regulated by the FDA?
The FDA regulates microneedling products that are medical devices to make sure they are safe and work as claimed. Not all microneedling products are medical devices.
Microneedling products that are medical devices
The FDA has cleared microneedling devices for use as a treatment to improve the appearance of facial acne scars, facial wrinkles, and abdominal scars in patients aged 22 years or older.
Most of the cleared devices are pen-shaped, motorized and penetrate the skin in order to change the structure or function of the tissue below. Because these devices may reach nerves, blood vessels and other parts of the skin, the FDA recommends you go to a health care provider with special training in microneedling.
The FDA has not authorized any microneedling medical devices for over-the-counter sale.
Microneedling products that are NOT medical devices
Generally, if a microneedling product does not have longer needles or sharp needles that penetrate the skin and only claims to facilitate exfoliation of the skin or improve the appearance of skin, it would not be a medical device regulated by the FDA. A dermal roller with short, blunt needles that only claims to help remove dead skin and smooth and brighten your skin would be an example. These products are more commonly sold for use at home.
What are the benefits of microneedling devices?
The FDA has cleared microneedling devices for use on women and men aged 22 and older as a treatment to improve the appearance of:
- Acne scars on the face
- Wrinkles around the eyes and other parts of the face and neck
- Surgical scars on the abdomen
What are the risks associated with microneedling devices?
Any microneedling device has the potential to cause side effects that last a short time or a long time.
Skin damage is a risk that commonly occurs with microneedling devices. The damage may include bleeding, bruising, redness, tightness, itching and peeling, and these typically go away without any treatment after a few days or weeks.
Less common risks include stinging or itching when cosmetics or other skin care products such as moisturizers and sunscreen are applied, dark or light spots on the skin, lines on the face, a flareup of cold sores, swollen lymph nodes, and infection.
Be aware, microneedling may not always result in the desired aesthetic outcome and it may take more than one procedure to get the results you are seeking. In addition, any improvement in appearance may be temporary and maintenance may require additional procedures over time.
The FDA’s web section on microneedling devices has more information on risks.
What safety tips should I know before microneedling?
Microneedling is not suitable for everyone, so it’s important to talk with a health care provider. Be aware:
- Numbing medication (topical anesthesia) is typically applied to the skin before the procedure to help reduce pain during microneedling. Tell your provider if you have any allergies or sensitivities to numbing medications.
- Microneedling can cause bleeding so it may not be suitable for people with clotting or bleeding disorders, or who take medicine to thin their blood.
- You may want to avoid microneedling if you have conditions that affect your skin, such as eczema or diabetes, or if you have a weakened immune system.
- Ask your health care provider how the microneedling device is cleaned between patients. Microneedling devices that have reusable parts should be cleaned and disinfected according to the device instructions.
- Ask your health care provider if a new needle cartridge is used for each patient and for each treatment session with the same patient. Re-using cartridges can cause or spread infection.
- Ask your health care provider how to care for your skin after the procedure, as your skin may be more sensitive to the sun and to skin care products like sunscreen, makeup, and moisturizers, especially products that contain retinol, glycolic acid, menthol, capsaicin or alcohol.
- If you have a microneedling product for home use, clean it between uses as directed by the manufacturer. Do not share these products with other people to avoid possible infection or spreading disease.
What else should I know about microneedling?
The FDA has cleared devices with microneedles that deliver radiofrequency (RF) energy (heat) for the treatment of facial wrinkles and other treatments. There are different risks with RF microneedling devices, as they work differently.
You may have heard about microneedling being used to combat hair loss. While clinical studies may be going on, the FDA has not cleared any microneedling devices for this use.
You may be aware of microneedling products used along with creams, ointments, other drugs or cosmetics, or platelet-rich plasma. The FDA has not cleared any microneedling devices for use with another product, which means the FDA hasn’t reviewed the safety or effectiveness of combining microneedling devices with other products. The FDA’s web section on microneedling devices has more information on recommendations for patients and health care providers.










