Until recently, the only effective procedure available to the individual wanting appearance enhancement was a face lift. Throughout the past few years, treatments featuring Botox and fillers, as well as laser and light procedures—in conjunction with good skin care—have revolutionized the ability to rejuvenate an aging face.
Statistics
According to a consumer survey by the American Society for Aesthetic Plastic Surgery (ASAPS) of 1,000 American households in 2007, 83% of women and 78% of men would not be embarrassed to undergo cosmetic surgery. This has been a major shift in society’s views toward cosmetic procedures. It has become trendy to openly contemplate procedures with peers, discuss a physician’s techniques and compare results. This trend, however, is occurring in regard to noninvasive, nonsurgical procedures. Recent statistics from the ASAPS reveal that, of 11.4 million procedures performed, 17%—or 1.9 million—were surgical, such as face lifts, eyelid lifts and liposuction, while 83%—or 9.5 million—were nonsurgical, such as the injection of Botox and fillers, and laser and light-based treatments.
Fillers and injectables
While face lifts are surgical procedures associated with anxiety, high cost,and significant recovery periods, filler and Botox treatments are relatively simple, safe, quick, relatively inexpensive and, in many instances, without downtime. Many individuals who would never consider a cosmetic surgical procedure are happy to have a noninvasive procedure. Because of this, the potential pool of candidates has increased dramatically.
At the same time, there have been incredible technological developments related to these noninvasive options. Product and equipment manufacturers have initiated more aggressive marketing approaches, using increasingly sophisticated and expensive direct-to-consumer television and print campaigns.
History
Since the early 1900s, there has been an interest in finding filler substances to enhance the face. The goal was safe and effective tissue augmentation. The early history, however, is full of examples of poor results and terrible reactions.
Injectables’ past begins when German physician Franz Neuber used fat from patients’ arms to fill facial defects in 1893. Foreign substances, such as paraffin and silicone, were used throughout the early-to-mid 1990s. Paraffin induced foreign body reactions and its use as a filler was discontinued. Impure and adulterated silicone has been used quite extensively, resulting in allergic reactions, as well as foreign body responses. In the past few years, the U.S. Food and Drug Administration (FDA) approved two different pure medical-grade liquid silicone products for ophthalmic use. Although not yet approved as a filler substance for aesthetic procedures, clinical experience and results from unpublished studies are encouraging.
Neuromodulators
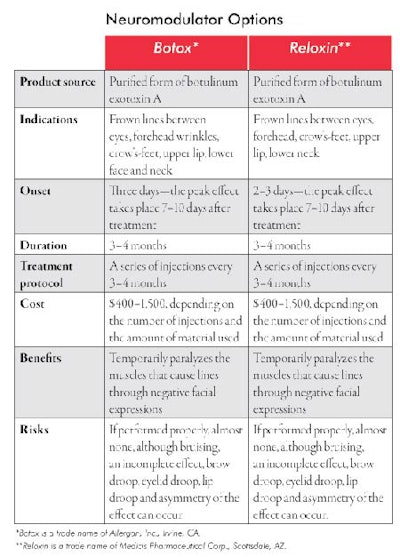
Neuromodulator is the generic name for a group of toxins that selectively block nerve innervations of skeletal muscles. Botulinum exotoxin A, known by the trade name Botox*, is, at this time, the only approved neuromodulator for cosmetic use in the United States. It was accepted by the FDA for cosmetic use in 2002. The year it was introduced, it became the No. 1 injected cosmetic material in use, and one of the most commonly performed cosmetic procedures by physicians and nurses. According to the ASAPS, more than three million people in the United States received a Botox injection in 2006. It is a highly diluted form of neurotoxin that selectively and temporarily paralyzes voluntary skeletal muscle function and it represents only one of a group of neuromodulators. They are designated as botulinum exotoxin A though F. Botox is one of the type A exotoxins. Reloxin**, a different type-A toxin, is presently under review in the United States and is awaiting approval by the FDA, which is expected to happen sometime in late 2008. See Neuromodulator Options for more information about the two types. Myobloc is a type-B botulinum toxin that is not yet approved for cosmetic use, is slightly more painful upon injection and has a shorter-term effect than that of Botox. Other exotoxins are under investigation in the United States and will likely be approved during the next several years.
Fillers
Fillers are biomaterials that can safely and effectively fill creases, lines and areas experiencing volume loss due to facial aging and skin conditions associated with tissue loss. These materials are best classified as either temporary, semi-permanent or permenant, which in turn is defined by the makeup of the substance.
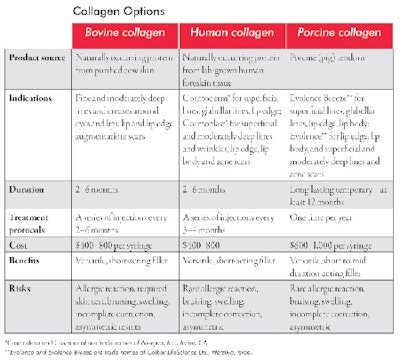
Temporary fillers. Collagen was the first approved filler in the United States and has been in continuous use since the early 1970s. Because it doesn’t last very long, it has been mostly replaced by other longer-lasting, safe and effective fillers, such as hyaluronic acids. Approved collagens are either bovine or human in source. Currently, porcine collagen, derived from pigs, is awaiting imminent FDA approval. See Collagen Options for more information about the various types. The advantage of human and porcine collagen is the very low risk of allergic reactions when compared with bovine collagen, which requires a skin test to rule out allergy.
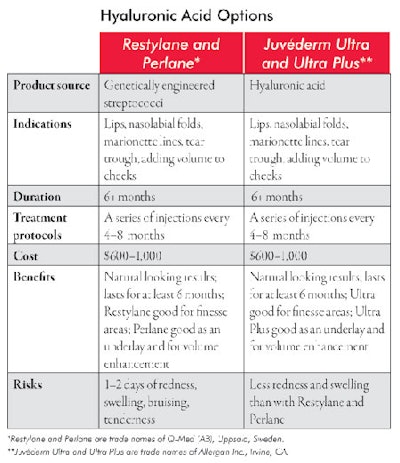
Hyaluronic acid. Hyaluronic acid, a normal constituent of the dermis, is a complex sugar that makes up a significant portion of the substance of the dermis where collagen and elastin fibers, vessels, nerves and apendageal structures lie. It makes for a versatile filler because allergic reactions are uncommon, it can be used to fill small, large, shallow and deep defects, and it lasts, on average, at least six months. It is effective as soft tissue filler for fine and moderately deep lines, wrinkles and for volume replacement in the face, cheeks and lips. The moldability of the products and natural look of the results make it an appealing filler, and, because allergic reactions are so uncommon, the patient can be treated on the same day as the initial consultation. See Hyaluronic Acid Options for more information about the different available products.
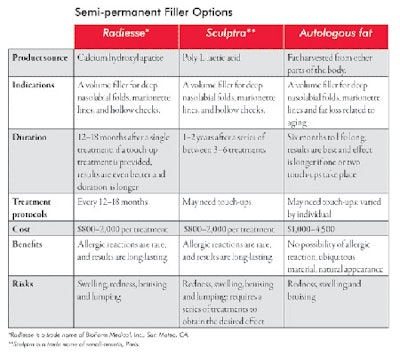
Semi-permanent fillers. Semi-permanent fillers fall between a short, lasting injectable and a permanent injectable, depending on the type of filler and the quantity used. The duration of its effectiveness is somewhere between 6–12+ months. The source of semi-permanent fillers is either natural (purified hyaluronic acid with naturally occurring complex sugar and fat) or synthetic (biodegradable polymer of polylactic acid and hydroxyl apatite). See Semi-permanent Filler Options on Page 62 for more information about the various options available.
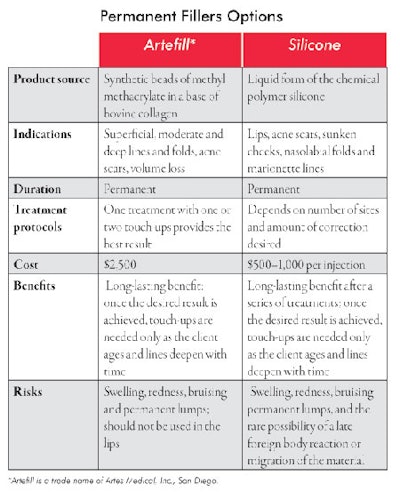
Permanent fillers. Permanent fillers have permanent side effects. These materials must be used judiciously and with the right candidates. It is best to first treat these individuals with temporary fillers to be sure they like the result before injecting a permanent filler. The two presently available in the United States are silicone and Artefill. The former is approved for injection in the eye to treat recurrent retinal detachment. When used as a filler, it is considered an off-label use.
Artefill is the first permanent injectable filler approved by the FDA. It consists of synthetic beads of methyl methacrylate in a base of bovine collagen. After injection, the bovine collagen slowly dissipates leaving behind the permanent portion of the product—the synthetic beads of methyl methacrylate. Due to its permanency, it is recommended to do a series of injections for the gradual filling of defects in order to eliminate overcorrection and lumps. Because its source is bovine collagen, allergic reactions are possible. See Permanent Filler Options for more information about the two types.
Techniques
Physicians and nurses must select which material and technique is the best suited for a patient’s particular type of defect, its location, and its desired results. Some want a natural look, while others prefer a more noticeable change. Technique is important in producing the desired results when using injectables. The experience and artistic ability of the physician certainly contribute to satisfying results.
Linear threading. The length of the needle is injected into the wrinkle’s center. The material is injected either as the needle is advanced (anterograde injection) or while it is gently pulled back (retrograde injection); or it can be injected in both directions.
Serial puncture. Multiple punctures are made along the length of the defect, and small amounts of the filler are injected in each site.
Regardless of the technique used, it is best to gently massage the material to ensure even placement of the filler.
Indications, benefits and risks
The general indications for the use of injectables are the patient’s desire to enhance their appearance, whether by eliminating the visible signs of aging or improving the appearance of a tired, aged face. These manifest through fine lines and wrinkles, smile and frown lines, crow’s-feet, nasolabial folds, forehead wrinkles and visible lip aging.
As for benefits, injectables are readily available in physicians’ offices, easy to administer, and results can be quick and effective.
As with anything in the medical field, there can be side effects—most are quite minimal, though in rare cases can be more severe. These can include headache, allergic reactions, swelling, redness, tenderness, bruising, lumpiness, and an incomplete or asymmetrical result.
Combination therapies
The visible signs of aging are due to a combination of factors, including the appearance of lines and wrinkles, volume loss and sagging skin. Therefore, it sometimes becomes essential for physicians and nurses to employ a variety of treatments and techniques to address a patient’s various concerns. For example, it may be recommended to combine Botox, a filler or several fillers, skin care, and laser or light treatments for an individual to accomplish an improvement of the entire canvas of the face instead of a simple spot improvement.
Due to both the extraordinary popularity of injectables and consumers actively driving the industry, manufacturers are clamoring to develop the next blockbuster neuromodulator or filler. Several new neuromodulators await FDA approval, and many new fillers already available in Canada and Europe are under investigation in the United States. Some will be terrific products and others, less so. The injectable arena is a very exciting one in which tremendous developments are awaited with anticipation.
The future
Physicians are looking for ways to get the extreme results of a face lift, but with a more natural look and without an extended recovery time, and the negative side effects of pain and scarring. The options for achieving this will continue to expand because manufacturers are determined to keep supplying more injectables as the demand for them continues to increase. The ability to have remarkable results and client satisfaction without surgery will promote the continued development of materials and nonsurgical procedures that are new, improved and, most importantly, long-lasting.