Sunscreens are a vital tool in the fight against skin cancer and are highly effective in preventing skin aging caused by the sun (e.g., wrinkles, pigmentation irregularities, dehydrated and sagging skin). As more consumers and wellness/health care professionals understand sunscreens, appreciation grows for the importance of these products in protecting our health. It is important for the esthetician to have a fundamental knowledge of sunscreens to recommend appropriate protection for their clients. This ensures compliance and most importantly, adequate protection.
This article will explore some basic considerations behind sunscreen products, such as the two main categories of marketed sunscreens, formulation architecture and delivery systems—the anatomy of a sunscreen. Keep in mind that the U.S. Food and Drug Administration (FDA) regulates all sunscreens, including any cosmetic or personal care product labeled with an SPF, as over-the-counter (OTC) drug products. This requires all applicable sunscreen products to conform to OTC drug regulations, as far as labeling, testing, permitted active ingredients, etc.
Intentional vs. Incidental Exposure
Products that contain sunscreening ultraviolet (UV) filters intended to provide sunburn protection can be divided into two basic categories. The first is when there is “intentional” exposure to sunlight for extended periods of time. This is typically associated with recreational activities such as swimming, hiking, sports activities, etc., with exposure to sunlight during the period of highest sunburn potential—10 am to 4 pm. These activities often induce sweating and may also include exposure to water for a significant amount of time. Sunscreen products for these activities are designed to resist removal by sweat or water by incorporating polymer substances and emulsifiers that lock the sunscreen filters onto the surface of the skin.
The second category of sunscreens typically performs a primary function other than sun protection, and is therefore intended for “incidental” sun exposure. The primary functions may be for moisturization; to cosmetically improve the skin’s appearance and even out skin tone, such as tinted foundations; or to correct blotchy or uneven skin tone. Typically these products are for the face or “spot” use, such as on the hands, arms or décolleté, which are exposed and the most visible during the day.
These “cosmetic” products containing UV filters and providing a measurable sun protection factor (SPF) are also categorized by the FDA as drug products, and require the same exact labeling instructions and description as the “intentional” or “recreational” sunscreen products. “Incidental” sun exposure products typically have a lower SPF as they are intended to be used only for short periods of sun exposure. Yet, they fulfill a critical role in providing the constant protection that is vital to maintaining healthy skin against cancer, wrinkles and age spots.1
Fundamentally, the two types of products are formulated with the same basic categories of ingredients, with the exception of film-forming polymers in the first category to provide protection against sweating and water resistance. Let’s look at the “skeleton” of these products to see how they are constructed, how they are similar, and how they differ.
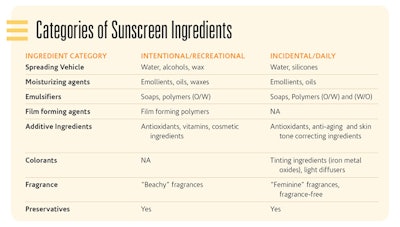
The primary categories of ingredients are: spreading vehicles, emulsifiers, film-forming agents, additional benefit agents, colorants, fragrances and preservatives. In the Categories of Sunscreen Ingredients sidebar, examples of ingredients are provided for both the intentional, recreational sunscreens and the incidental, daily use sunscreens.
 Sunscreen Active Delivery Systems
Sunscreen Active Delivery SystemsProducts containing sunscreen filter actives are available in a wide variety of delivery systems designed to fit specific use conditions and consumer preferences. Compliant sunscreen protection requires convenience and product elegance to ensure the products are used regularly and in sufficient quantity. The effectiveness of a sunscreen-containing product is critically dependent on the amount used and the way the user applies it to their skin. Several studies2, 3 have shown a direct linear relationship between the amount of sunscreen applied to skin, and the resulting SPF. In short, if a user applies only half of the recommended amount that was used during clinical SPF testing of the sunscreen (2 mg/cm2), then the resulting SPF the user actually gets will be half of the amount stated on the product label.
Convenient packaging can support user compliance by making sunscreens readily available, such as a in stick applicator that can be carried in a purse or pocket, a spray product that is “easy” to apply to wiggling children, or a lotion bottle with a pump dispenser placed on a dressing table or makeup counter.
Small packages of lotion or powder products with sunscreen actives can also be carried in a purse for touch-up applications, or tucked away in the glove box of a car for access at a moment’s notice.
Each of these product forms has different vehicle constituents to fit the storage and use conditions, but all have the same intent—to deliver a thin film of sunscreen filter actives to the skin and provide an optical barrier to UV rays from the sun or other UV light sources, such as indoor fluorescent lighting.4 Let’s look at each vehicle type and examine the components.
Lotions and Creams. Lotions and creams comprised the majority of the sunscreen product category until the mid-2000s. Both of these forms are mixtures of water and oil, with the sunscreen filters dissolved into one or both of the two phases. Lotions and creams are typically oil-in-water (o/w) emulsions, which can be described as droplets of oil swimming in a sea of water, held in a stable matrix by ingredients called emulsifiers that physically and chemically maintain equilibrium between the oil droplets and the sea of water. Without the emulsifiers and physical energy used to form the emulsion, the two phases would separate into a layer of oil floating on a layer of water—like your oil and vinegar salad dressing before you shake it.
After the lotion or cream is spread onto the skin, the water evaporates and the emulsion “breaks,” leaving the layer of oil containing the sunscreen actives to spread and remain on the surface of the skin.
Cream products are simply “thicker” versions of lotions, where more viscous oils, waxes or thickeners are added to make the product more moisturizing. Water-in-oil (w/o) emulsions are also possible, wherein you can imagine water droplets floating in a sea of oil. These “reverse” emulsion products tend to be heavier to spread on the skin and slower to dry down, as they typically have a much higher proportion of oil to water, compared with the more common o/w products that are typically 60% or more water. W/O products can leave a thicker oil film on the skin surface and are naturally more water resistant. However, the trade-off is they can be considered more “greasy” to the touch.
There is no easy way to read a product label and determine if it is an o/w or w/o emulsion. A simple test is to put a drop of the emulsion product in a glass of water and see if it easily disperses with slight agitation, or forms tight balls. If it disperses, it is an o/w emulsion; if not, it is a w/o emulsion.
Both emulsion types are effective vehicles for delivering sun protection. It is simply a matter of consumer preference for the product type based on its esthetic properties, the level of moisturization desired and the level of protection perceived.
Spray products. In the mid-2000s, spray sunscreens became a popular format and remain the highest selling category of the “recreational” sun care market, with more than 50% of total sales. The vast majority of spray sunscreen products are solutions of alcohol containing UV filters, with some additional oils to form the film layer on the surface of skin. The alcohols provide a quickly evaporating vehicle, as well as a solvent that conveniently solubilizes and distributes the sunscreen actives in the container to evenly disperse them on skin. While concerns have been raised by the media and consumer advocates regarding the safety of inhaling of spray sunscreens, this risk is mitigated by the use of spray systems that provide droplet sizes in excess of 10 microns in diameter—a size shown to prevent deep inhalation into the lungs.
Stick products. Stick sunscreens are a convenient dosage form for spot applications such as the nose, ears and around the eyes. These consist of various moisturizers or emollients that can solubilize oil-soluble UV filters and provide a vehicle for any particulate sunscreens; i.e., those whose particles physically block, scatter and reflect UV, including titanium dioxide and zinc oxide. Also incorporated are waxes, thickening agents and polymers to help the product hold its shape at temperatures above around 120°F. When rubbed across skin, it leaves a continuous film of compounds containing the sunscreen filters that resists wash-off.
Powder products. Facial powder sunscreens consist primarily of titanium dioxide and zinc oxide powders that provide UV protection, together with emollient binders and mineral pigments that provide color and glow. These products help to even out and brighten skin tone while providing protection against damaging UV radiation.
Future Skin Health
Scientific data overwhelmingly tells us that daily sunscreen use makes a profound improvement in skin health. Establishing and practicing sun-safe habits from an early age is imperative to protecting against skin aging and cancer. All individuals should use sunscreen and other sun protective measures every day—and it is important for you to understand how sunscreens function to not only better protect yourself, but your clients as well.
REFERENCES
- TJ Phillips, J Bhawan, M Yaar, Y Bello, D Lopiccolo and JF Nash, Effect of daily versus intermittent sunscreen application on solar simulated UV radiation induces skin responses in humans, J Am Acad Dermatol 43(4) 610-618 (2000)
- R Bimczok et al, Influence of applied quantity of sunscreen products on the sun protection factor–A multicenter study organized by the DGK Task Force Sun Protection, Skin Pharmacol Physiol 20 57–64 (2007)
- H Ou-Yang, J Stanfield, C Cole, Y Appa and D Rigel, High SPF sunscreens (SPF>70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen used application amounts, J Amer Acad Dermatol 67 6 1220–1227 (2012)
- CA Cole, PD Forbes, RE Davies and F Urbach, Effects of indoor lighting on normal skin. Annals NY Acad Sci 453 305316 (1985)