Sun protection has come a long way since the Coppertone girl of the 1950s. Although sunburns then were no doubt as painful and unhealthy as they are today, sunscreen was definitely not viewed as a necessity. It was just a lotion every mom had in her beach bag and only used after her kids were already burned.
Well, times have certainly changed. Sun protection is now considered a daily-use item because it is well-known that UV rays cause premature aging, skin damage and even cancer. The stuff is no longer looked at as something you need use only during a day at the pool or the beach. Sunscreen can be found in everything from facial moisturizer to lip balm ... and is no longer relegated to the bottom of the bag.
The evolution of sunscreen
Sunscreens may not have been a common household item decades ago, but the product does have a long history. The first sunscreen is thought to have been developed by chemist Franz Greiter in 1946. As the story goes, Greiter acquired a sunburn while climbing Piz Buin mountain in the Alps, which led him to create a sun-protection product, aptly named Gletscher Crème or Glacier Cream, which is believed to have had a sun protection factor (SPF) of 2. Consequently, Greiter also invented SPF—the standard of measuring sunscreen effectiveness—in 1962. His formula, sold under the brand name Piz Buin, is still on the market today.
Remember the little Coppertone girl with the puppy pulling on her bathing suit? This popular product started out as a gooey red substance called Red Vet Pet. Created by pharmacist Benjamin Green in 1944, Red Vet Pet stood for “red veterinary petroleum” and had a sticky, red petroleum jellylike consistency. Not exactly a poolside staple by today’s standards, but sales of the formula took off when it was purchased by Coppertone, mixed with cocoa butter and coconut oil, and then marketed as Coppertone and Ban de Soleil.
Thank goodness we are not limited to the sticky redness or greasy whiteness of sunscreens from years gone by. Today’s sun protection products are not only super effective at protecting against UV damage, but invisible and comfortable on the skin. Although variety may not be the issue, wild claims of safety and effectiveness have led to recent consumer confusion.
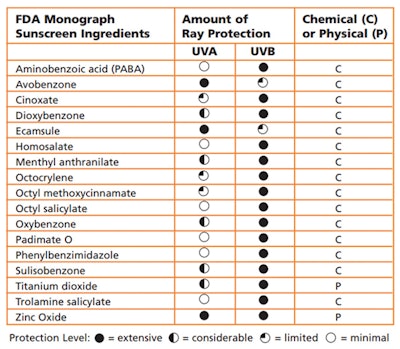
How much SPF is enough? Is a higher SPF better? What about UVA and UVB protection? These are only some of the questions consumers are asking. It is now known that UVB rays cause sunburns, but UVA rays are responsible for skin cancer and premature aging. So, it is important that both you and your clients become aware of which products provide this broad-spectrum protection. (See Sunscreen Ingredients Chart.)
Demystifying SPF
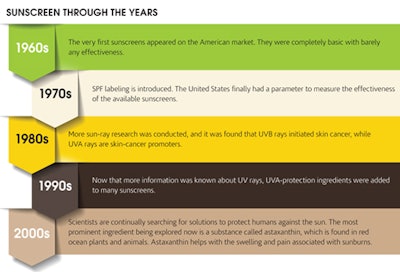
Generations ago, the best known way to protect yourself from the sun was to wear long-sleeved clothes or a bonnet. The effects of the sun were clear when people ventured into the beaming light with no protection and ended up with bad visible burns. Now, the science and understanding of the sun’s rays and the effect they have on skin has advanced to the point where only sunscreen needs to be applied to provide your body with the protection it needs. Throughout the years, sunscreens have taken a dramatic turn for the better when it comes to skin protection. (See Sunscreen Through the Years.)
Although the SPF on a bottle of sunscreen is commonly referred to when shopping for sun protection, few clients actually know what the number system means. The SPF of a sunscreen tells you two things: how long you can stay in the sun and how well the formula filters the sun’s rays. First, if you take the amount of minutes you can stay in the sun before turning pink and then multiply that number by the SPF rating, you get the amount of time you can spend in the sun using that sunscreen. For example, if you are normally able to stay in the sun for 10 minutes without burning and the SPF of your sunscreen formula is 20, and it is applied correctly, your sunscreen will protect you for 200 minutes before your skin starts to burn. How well the formula filters the suns rays goes as follows.
- SPF 2 blocks about 50% of UVB rays.
- SPF 10 blocks about 85% of UVB rays.
- SPF 15 blocks about 95% of UVB rays.
- SPF 30 blocks about 97% of UVB rays.
Above SPF 30, the increased percentage protection is minimal. Instead, there is a longer period of time before sunburn takes place. Regardless, experts do recommend you re-apply at least every two hours.
A chronology of the FDA and sunscreen safety
The marketing and labeling of sunscreen has created much consumer confusion over the years, and a proposal to clarify sunscreen marketing has been in the works by the U.S. Food and Drug Administration (FDA) for decades. And as of 2013, it has finally been implemented.
In 1978, the FDA took limited steps to regulate the safety and effectiveness of sunscreens, and they were mainly focused on SPF testing and labeling. Although not all steps were enforced, the original proposal stated: “In the long run, suntanning is not good for the skin.” Who knew? Then in 1988, the FDA approved the UVA-only sunscreen ingredient avobenzone. Until this time, other approved sunscreens were UVB filters only.
In 2006, the FDA missed a deadline set by Congress for the approval of proposed sunscreen guidelines. The FDA finalized the proposal a year later. In 2010, with the guidelines still not approved, the target date was yet again pushed back.
In the summer of 2011, more than 30 years after its first stab at sunscreen regulation, the FDA set down legally binding rules on the marketing of sun protection products. Not so surprisingly, the proposed approval date set for June 2012 was set back to Dec. 17, 2012, but is now in full effect for all but manufacturers of drug products with annual sales below $25,000. These smaller manufacturers must comply by Dec. 17, 2013. These new FDA rules will help to clear up confusion over the following issues when purchasing sun protection products and include the following mandates.
Broad-spectrum. A sunscreen must meet broad-spectrum testing, which measures the UVA protection in relation to the UVB protection in a product. Sunscreens that pass the broad-spectrum test may be labeled “broad-spectrum,” indicating that they protect against both UVA and UVB rays. Only sunscreens with an SPF of 15 or higher can claim to reduce the risk of skin cancer or premature aging of the skin.
SPF. This is a measure on how long a sunscreen will protect skin against sunburn. The FDA has proposed that SPF will be capped at 50+ unless the manufacturer can provide information that supports a higher SPF number. SPF higher than 50 has been shown to offer minimal additional protection compared to sunscreens with SPF 50 and under.
Waterproof terminology. The term “waterproof” will be a thing of the past. New terms to be used are “water-resistant” and “very water-resistant,” determined after testing. “Waterproof,” “sweatproof” and “sunblock” are history.
Sun safety claims. Sunscreens can no longer state that they are immediately effective or provide more than two hours of protection unless approved as such by the FDA.
Type. Wipes, towelettes, powders, body washes and shampoo containing sunscreen will not be eligible for the new labeling without individual product approval.
A remarkable transformation
From thick, red cream to transparent, all-natural mineral protection, sunscreen has been through a remarkable transformation in the past century. Better UV protection and healthier ingredients make the sunscreens of today not only more effective, but safer to use.
GENERAL REFERENCES
yourtrainer.com/fitness-guides/danger-chemical-based-sunscreens
www.wisegeek.org/what-is-the-history-of-sunscreen.htm
heartspring.net/skin_care_skin_cancer_sunscreen.html
www.sunscreen.info/history
www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm258468.htm
en.wikipedia.org/wiki/Sunscreen
(All accessed on Mar 14, 2013)