Humans are quite a bit more than what they eat, but surely what they eat has a significant effect on their lives. All living organisms need energy to live, and that is obtained from food. It also supplies the essential building blocks of the body, enabling growth from infancy to adulthood and beyond. It is both a complex and fascinating story about how the physical features and the developmental history of humans have been shaped by what, and how early man consumed.
Primitive man
The time line of history is a long one, but for mankind, it is relatively short, depending on who you believe. Two million years is a good estimate, with Homo sapiens going back a million years.1 Others say it should be only about 500,000 years.2 Because man is a primate, he is a predator who looks for prey. Prey are animals that mainly eat grass and have eyes on the sides of their heads, usually with long necks and skulls to reach the ground, chopping, chewing teeth—but no canine teeth—and a very long and complex digestive system. Predators who feed on prey have short necks, powerful jaws for killing and ripping, crushing teeth and eyes that face forward. Both types of animals have acute senses of smell and hearing. Man can be both predator and prey.
Primitive man was mainly a meat eater. Small animals at first, such as bugs and mice, but as he developed, he chose larger animals. Of course, before the discovery of fire, all food was eaten raw. There were advantages and disadvantages to this practice. The upside was that he needed no supplements; all the vitamins and minerals were supplied courtesy of his victim. The downside was the meat did not last long because it decayed quickly and contained bacteria, parasites and heaven only knows what else. Primitive man also ate grass, tubers, nuts and berries. His digestive tract most likely could not handle uncooked grains, but the advent of fire solved that problem.
Approximately 40,000–50,000 years ago, a few smart early people noticed that if they put seeds into the ground, they no longer needed to go gather seeds far from home. Farming started as a primitive practice, but rapidly grew during the next 20,000–30,000 years. Animals were domesticated and houses were built, first as crude huts, but later as more elaborate dwellings. Society was born—all that was lacking was the invention of politics, which surely came with early civilization. Notice the flow of time and the decreased need for maximum effort on the part of mankind. With abundant food, clothing and shelter, life changed and with it, so did man’s need to adapt to a new lifestyle.
Mankind originally may have roasted food, and later used a boiling technique.Possibly the net result of plentiful cooked food was the onset of arterial disease and associated obesity. The human diet today includes overeating and many junk foods. Because of this, metabolic diseases have hit humans pretty hard, especially cancer and atherosclerosis, which is often linked to diabetesa.
Food and energy
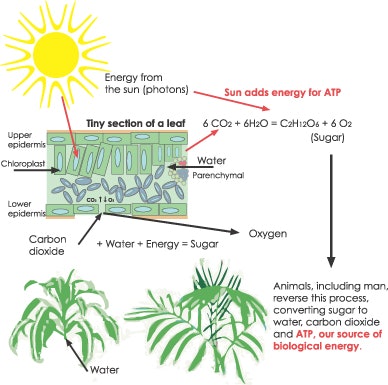
Without food, of course, people would die. Energy is needed in the form of adenosine triphosphate (ATP) every second of life. A fundamental physical law states that energy can neither be created nor destroyed, but only changed from one form to another. It is also known that all the energy on the Earth comes from the sun in the form of radiant—or electromagnetic—energy, which includes light at all wavelengths. From the sun, energy is captured by plants and locked into chemical bonds in the form of sugar molecules during the process of photosynthesis. Animals eat the plants and convert the energy into meat. Humans eat the meat produced by animals, releasing the stored energy and capturing it on their ATP molecules to be used to run the thousands of physical and biochemical reactions occurring in their bodies every minute. This process of energy production and utilization is related in a subtle way to the aging process. To get the full picture, see Figure 1 to trace the pathway from the sun to ATP.
Energy and aging
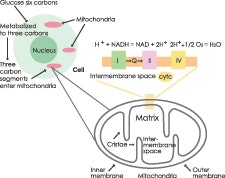
If you are not familiar with mitochondria in the cell, you should be. Mitochondria are rod-shaped organelles that are about one micrometer in length with a double membrane. A cell’s specific function and energy needs determine the number of mitochondria per cell. For example, the number of mitochondria in the liver are about 1,000–2,000 per cell, making up 20% of the cell volume.3 The inner membrane is folded into a form known as cristae that is actually extended into the inner membrane called the matrix, and between the inner and outer membrane is the intermembrane space. Because the mitochondrion has its own circular DNA (mtDNA), there are four to five copies of mtDNA that encode some of the proteins of the electron transport chain (ETC). It is possible that mitochondria were primitive prokaryotic bacteria that were engulfed by an eukaryotic cell, somehow developing a symbiotic relationship.
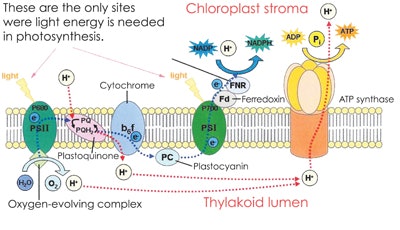
Mitochondria are the organelles in the cell that make most of a person’s ATP. In Figure 2, the process of making ATP in the cell has been outlined, including the process in the mitochondria. This is the chloroplast stroma where the actual ATP of the leaf is made. This is a complex diagram, so just look at the two sites where the energy of sunlight is needed, and then look at the end of the diagram where ATP is produced in the conversion of hydrogen ions to ATP. This structure is ATP synthase, which is very similar to what humans have in their mitochondria. Notice also that at the first molecule where light is needed, water is being split to oxygen and hydrogen. You will see that all the energy production in the mitochondria requires the presence of oxygen, and with a constant supply of molecular oxygen, you have the potential for free radical damage.
Mitochondria generate the majority of energy from nutrients, but in the process, they generate unstable chemicals that harm both the mitochondrion itself and other components of the cell. This resulting damage is thought to play a important role in aging.
ATP and aerobic respiration
Mitochondria use oxygen to perform aerobic respiration, which generates ATP from nutrient molecules. Three key components of aerobic respiration are glycolysis, the Krebs cycle and the ETC. See Figure 3.
In glycolysis, a single molecule of glucose is metabolized into two molecules of pyruvate, a three-carbon compound generating two ATP and two nicotinamide adenine dinucleotide (NADH) molecules. Pyruvate is converted into acetyl-CoA, generating one molecule of carbon dioxide that enters the Krebs cycle to generate one molecule of ATP and flavin adenine dinucleotide plus hydrogen squared (FADH2), and three molecules of NADH per acetyl-CoA. It is these NADH and FADH2 molecules that serve as electron carriers to transfer electrons generated by glycolysis and later by the Krebs cycle into the ETC.
In the ETC, the inner membrane creates a pH gradient with these electrons across the inner membrane. Next, the membrane proteins pump protons from the inner matrix into the intermembrane space. It is this chemosmotic gradient that serves as an energy reservoir to drive the formation of ATP as protons are pumped back into the inner matrix via a membrane protein called ATP synthase. Each glucose molecule yields 32 ATP via the ETC, in addition to two from the Krebs cycle and two from glycolysis to total 36 ATP molecules generated via aerobic respiration. This is an enormous increase over anaerobic respiration, and it made multicellular life possible.
Aerobic respiration and aging
A continuous stream of molecular oxygen will create plenty of unstable reactive oxygen species (ROS) capable of damaging many types of cellular components, and much of this oxidative damage will accumulate throughout time and play a significant role in aging. Because mitochondrial DNA exists in the inner matrix, and they are closer in proximity to the inner membrane where high energy electrons form unstable compounds, the mtDNA has a high chance of being damaged by ROS-causing mutations of mtDNA that can result in the manufacture of mutant ETC proteins leading to the leaking of more electrons and thus more ROS. This is the basic concept of the defective or damaged mitochondrial theory of aging.
There is a lot of evidence that suggests that mitochondria are implicated in the aging process. Mainly, the loss of the integrity of the mitochondrial genome is thought to be the culprit in aging. One component of DNA replication involves a proofreading ability of mitochondrial DNA to make sure it is normal before it is reproduced. The agent responsible for this is mitochondrial polymerase and in one study it was removed in mice models. The mtDNA mutations that were reproduced caused the development of a mouse that resembled the physical signs seen in premature aging.4 It may be that the accumulation of mtDNA mutations might be the result of a more fundamental process that can bring about aging changes rather than the actual defects in mitochondrial genome causing aging.
Diet, energy and aging
It has been known for more than 70 years that dietary restriction can increase life span, although scientists are still not certain about how this is achieved. It was first suggested that calorie restriction (CR) exerts its beneficial effects by slowing carbohydrate use, respiration and the rate of damage produced by ROS. This may not be the case, however. A careful examination of the physiology of CR has shown that rather than slowing respiration, CR actually increases mitochondrial function. Also, CR appears to be a regulated—rather than a passive—process, requiring protein, such as sirtuins, to regulate and exert its effects.5 Sirtuins comprise nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylases that increase life span in yeast, the worm Caenorhabditis elegans and the fruit fly Drosophila.6 Sirtuins have roles in CR, but their connection to mitochondria is less clear.7
Although there is a lot of information about the benefits of CR for lower life forms, the whole mechanism is nowhere near completely explained. Certainly there is some activation of the brain and hormonal interaction. In some way, the mitochondria are changed. For one thing, they increase in number per cell when the supply of glucose is low. It may be a simple switching mechanism involving a very complex biological chain of events. For example, mankind is normally programmed to eat and to reproduce. Take away food, and reproduction is no longer given a priority; survival is the goal of the body. How long will the famine last? The body must be prepared for the long haul; it must be lean and trim and strong to withstand the rigors of survival during hard times. All of this can be directed by the brain once the message is given, “No more pigging out, cut back and survive!”
Mitochondria play an important role in aging, along with the sirtuins as anti-aging genes, and at least four of the seven mammalian sirtuin homologs have mitochondria-associated functions. At present, CR is the best intervention known to extend mammalian life span, and CR is associated with an increase in sirtuin levels and in mitochondrial components.
What is the best way to use this knowledge to help your clients stay healthy? First, suggest that they reduce food intake, at least 25% less, perhaps up to 40%. Far too many calories are consumed for the body’s needs, becoming wasted energy that damages the mitochondria. Second, antioxidants should be taken along with the CR diet. To this, I would recommend resveratrol at 100 mg to 500 mg daily. Obviously, suggest a moderate exercise program—walking and weight-lifting are two effective methods. Also, encourage clients to set goals to learn something new each day in order to challenge their brains. Less television and more books. Health is about mind and body. Your skin will reflect this inner health with an outer glow that will proclaim, “I am with it.”
REFERENCES
1. JC Calister, Brief Review in Earth Science, Prentice Hall, Englewood Cliffs, NJ, 188–189 242–243 (1993)
2. RE Leakey and R Lewin, People of the Lake: Mankind and its Beginning, Anchor Doubleday, New York, 252–253 (1978)
3. B Alberts, A Johnson, J Lewis, M Raff, K Roberts and P Walter, Molecular Biology of the Cell, Garland Publishing, Inc., New York (1994)
4. A Trifunovic, Premature ageing in mice expressing defective mitochondrial DNA polymerase, Nature 429 417–423 (May 27, 2004)
5. L Guarente, Sirtuins as potential targets for metabolic syndrome, Nature 444(7121) 868–874 (Dec 14, 2006)
6. S Imai, CM Armstorng, M Kaeberlein and L Guarente, Transcriptional silencing and longevity protein Sir2 is an NAD- dependent histone deacetylase, Nature 403(6771) 795–800 (Feb 17, 2000)
7. J Landry, et al., The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA 97(11) 5807–5811 (May 23, 2000)
FOOTNOTES
a. A really interesting book on this topic is Paleopathology at the Origins of Agriculture (Academic Press, 1984) by Mark N. Cohen.