Vitamin C, also known as ascorbic acid, is the prince of vitamins and the first dietary substance associated with curing a disease known as scurvy, a deadly and painful illness that was prevalent before James Lind, MD, discovered the cure in 1747. He established that the absence of a compound in the diet was the cause of scurvy. Lind died in 1794 at 78, and the following year, the British Admiralty adopted the use of citrus foods as the prevention of scurvy. In 1935, vitamin C was synthesized in pure form.
Vitamin C is also an important part of a variety of bodily functions, ranging from bone formation to scar tissue repair. It is the major water-soluble antioxidant, it destroys free radicals, it plays a critical role in hydroxylation reactions that are essential for the formation of collagen, and carnitine synthesis uses vitamin C as a reducing agent. It is directly involved in the formation of norepinephrine and serotonin, two important substances needed for the proper function of the nervous system; and it is also involved in the synthesis of hormones, hormone-releasing factors and neurotransmitters.
The chemistry of vitamin C
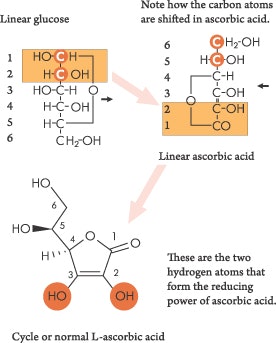
The chemistry of vitamin Ca and its role in the body can be more easily understood if you remember that it is a reducing agent. This means that it donates hydrogen to other compounds. Vitamin C is formed from glucose in the bodies of most animals, except primates and guinea pigs. Humans lack the enzyme L-gulonolactone oxidase, which converts glucose to ascorbic acid. Although other enzymes are also required for the conversion, this one is definitely lacking. Take a look at the vitamin C molecule in Figure 1. It is a simple sugar molecule with a slight modification. The important parts of this figure are the two OH groups (hydroxyl groups) on the vitamin C molecule. It is the loss of the hydrogen atoms from these OHs that makes vitamin C a hydrogen donor and, therefore, a reducing agent.
Vitamin E, also known as tocopherol, is an oil-soluble vitamin antioxidant and a reducing agent. It has only one OH group on the end, which is the active part of the molecule that donates the hydrogen atom in an antioxidant reaction. Now, here is the bad thing about vitamin E; losing a hydrogen from the hydroxyl group converts the molecule of vitamin into a quinone. So what, you say, is the big deal? Quinones are highly reactive molecules associated with neoplastic disordersb. The OH group is converted to carbonyl—that is to a -C=O—which can very easily react with proteins and make them nonfunctional. Along comes vitamin C and donates an H+ to the -C=O, changing back to the more beneficial -OH group. This is a very necessary function of vitamin C as a critical part of the antioxidant defense system.
Collagen formation
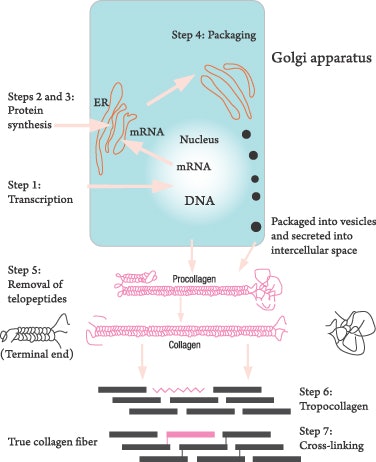
Collagen is the most abundant protein in the body; it makes up 70% of the dry weight of human skin and is a critical component of the vascular and muscular system. Vitamin C is essential to the formation of collagen. Although collagen formation is a complex process, the steps can be limited to only seven: four inside the fibroblasts and three in the cytoplasm. This process, diagrammed in Figure 2, is in a schematic form.
- In Step 1, transcription occurs, and vitamin C is needed. Transcription is the process by which deoxyribonucleic acid (DNA) opens from a double strand to two single strands to allow a copy to be made of the collagen code. The code is transcribed as ribonucleic acid (RNA) and then leaves the nucleus as messenger RNA (mRNA). The mRNA enters the endoplasmic reticulum (ER), which uses it as a template to build the collagen protein. In this first step, a polypeptide is synthesized in the ER. The polypeptide is the beginning of the collagen protein.
- In Step 2, the polypeptide is modified by a process called hydroxylation and glycosylation, which is essential for the formation of cross-linking in the next step.
- Step 3 produces the triple helix procollagen, which also occurs in the ER, but more in the rough ER rather than the smooth ER.
- The last intracellular formation of procollagen occurs in the Golgi apparatus in Step 4, the excretion step in which the procollagen fibril is prepared to be excreted from the fibroblast into the extracellular space for final assembly into collagen fibers. Check these steps carefully in Figure 2.
- In the extracellular space, the procollagen fibers undergo Step 5 in which the telopeptides, or terminal ends, are cut off by hydrolysis and are now called tropocollagen.
- In Step 6, the tropocollagen begins to self-assemble into a collagen fiber, but it is not mature collagen until Step 7.
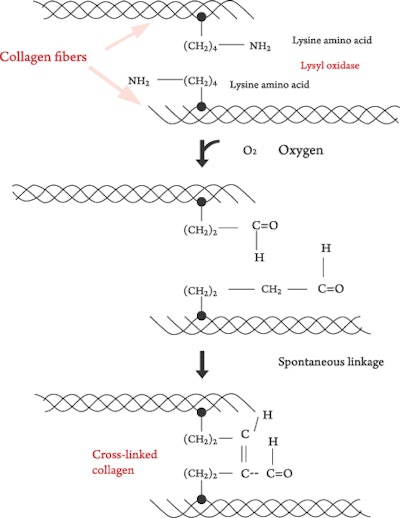
- Mature collagen is formed in Step 7, the stage of cross-linking. Vitamin C is used in the first step and in every step where hydroxylation occurs. See Figure 3 for a diagram of the hydroxylation process illustrating oxygen and ascorbic acid working on lysine to convert it to a carbonyl group, that is, an H-C=O group. Two of these groups combine and form a cross-linking of two collagen fibers. Ascorbic acid is essential for the initiation of transcription, the formation of the procollagen and the extracellular cross-linking of the collagen fibers.1
Vitamin C as an antioxidant
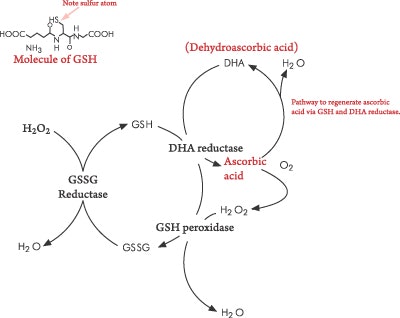
Since vitamin C is water-soluble, it is able to destroy free radicals before they can reach the cellular membrane. Vitamin E and glutathione (GSH) need vitamin C in order to be regenerated to their active forms. The relationship between vitamin C and GSH is unique because vitamin C reduces GSH back to its active form. Vitamin C can sequester the singlet oxygen radical and stabilize the hydroxyl radical, as well as regenerate vitamin E back to its active state. All of these functions are critical in preventing peroxidation of cellular lipid membranes.2 Now, on to free radicals.
First, free radicals need to be reviewed and named because there are many types of free radicals that can be formed in the body. For this article, they are limited to the oxygen-centered free radicals (ROS). The ROS include: the superoxide anion (O2-), the hydroxyl radical (OH ·), singlet oxygen (1O2), and hydrogen peroxide (H2O2).
Superoxide is formed when oxygen as O2 adds an additional electron. This results in the oxygen molecule having only one unpaired electron. One theory of aging is that, in the mitochondria, the O2- is constantly being formed and damaging the mitochondria.
Hydroxyl radicals are the most damaging radicals within the body, but they have a short life. Hydroxyl radical are formed from O2- and H2O2 via the Haber-Weiss reactionc. In the presence of H2O2, the metals copper and iron produce OH, and this is called the Fenton reaction. Hydrogen peroxide is produced in the body by many reactions, and since it can be converted to the highly damaging hydroxyl radical, it becomes dangerous. Catalase is the enzyme that destroys it so it can be excreted harmlessly as water. GSH peroxidase is an enzyme that converts GSH to oxidized GSH, during which H2O2 is converted to water. This is an important reaction because if H2O2 is not converted into water singlet oxygen, 1O2 is formed. Although singlet oxygen is not a free radical, it can be formed during radical reactions and cause further damaging reactions.
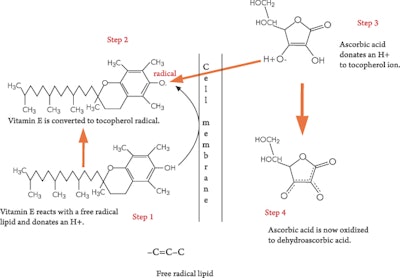
Check out the role of vitamin C in regenerating vitamin E. Figure 4 illustrates the reaction in which vitamin E donates an H+ to a peroxidated fat, and along comes vitamin C and donates an H+ to vitamin E to restore it to an active form.
GSH is a tripeptide containing three amino acids: glycine, cysteine and glutamic acid. It is a critical antioxidant that acts as an electron donor. GSH reduces any disulfide bond, an –S-S- group, within cytoplasmic proteins to cysteine groups, –S-H. Figure 5 shows this reaction in a simple form illustrating the electron transfer, as well as the structure of GSH. The importance of GSH lies not only in it being a critical antioxidant, but also that it is tied in with the regeneration vitamin C.
Other functions of vitamin C
Ascorbic acid is important in the metabolism of diverse amino acids that lead to the formation of norepinephrine, serotonin, homogenistic acid, carnitine, and hydroxyproline and hydroxylysine. It functions in enzymatic reactions and as a transport for the neurotransmitters, such as norepinephrine and serotonin. In fact, ascorbic acid is both in the production and protection of these neurotransmitters. Carnitine is found in the heart, muscles, liver and skin. One of the major functions of carnitine is to carry long-chained fatty acids into the mitochondria from the cytoplasm of the cell. This accounts for the lack of energy and lassitude seen in patients with scurvy.3
The liver serves as a major detoxifier in the body. Drugs and certain metabolites, such as bilirubin, are toxic if they remain in their original configuration. The liver uses many enzymes to modify these potentially dangerous chemicals, and these reactions often require a reducing agent, such as vitamin C. An example of this action can be found in cigarette smokers who usually have lower vitamin C levels than nonsmokers, even though they have the same vitamin intake.4
Perhaps the single most important function of vitamin C is in electron transport. On the molecular level, life is all about the transfer of electrons because when that process stops, it’s all over.
Finally, vitamin C interacts with mineral nutrients. Did you know that vitamin C intake has nutritional consequences on mineral nutrients, both inhibitory and enhancing type reactions. As an example, vitamin C in the diet can increase the absorption of intestinal iron and selenium, and it reduces the absorption of copper, nickel and manganese. Oddly, it has no effect on zinc, calcium and cobalt, or toxic metals, such as cadmium and mercury.5
Vitamin C in skin care
Using vitamin C effectively in skin care requires knowledge about its role in the skin. A very intriguing role for vitamin C involves the relationship between fibroblasts and keratinocytes.6 Vitamin C is suspected to exert some effect that controls proliferation and differentiation in keratinocytes. The addition of 25 µg/mL of vitamin C to a disordered arrangement of keratinocytes in a cultured epidermis was quickly followed by the disappearance of disorder and the appearance of a differentiation marker expression similar to that in normal skin. This data indicates that human fibroblasts and some modifier of proliferation and differentiation, such as vitamin C, are essential for epidermalization in reconstructed epidermis. Think about stretch marks—they are an epidermal manifestation of a dermal disorder in the fibroblasts.
Vitamin C, along with vitamin A, can reverse skin changes in both photoaging and chronological aging. It is known that plasma antioxidants are decreased in actinic keratosis and basal cell carcinoma, which is most likely due to long exposure to ultraviolet (UV) irradiation, a major factor in the etiology of these diseases. A decrease in blood levels of alpha tocopherol and GSH is also found in basal cell carcinoma.
Barrier function is an essential part of the job reserved for the stratum corneum. In a tissue-cultured study with keratinocytes, after two weeks of growth, an orthokeratinized epidermis evolved with the suprabasal layers exhibiting the normal differentiation markersd. Granular cells with keratohyalin granules and lamellar bodies, and corneocytes with cornified envelopes and tightly packed keratin filaments were present. Vitamin Csupplementation of the culture further enhanced the normal pattern of the stratum corneum and the number of keratohyalin granules present, and also, the quantity and organization of intercellular lipid lamellae in the interstices of the stratum corneum.7 These findings seen with vitamin C correlated with an improved epidermal barrier function as seen by transepidermal water loss values that were close to those of human skin.
Aging skin
Consider that the signs of aging skin are the same ones associated with loss of collagen production and damaged collagen. The use of vitamin C is a major line of defense, as well as a treatment of aging skin. Women experience a gradual loss of collagen integrity and production throughout the years that is associated with the flux in circulating collagenase due to cyclic levels determined by the menstrual cycle. Although this a new concept, it is nevertheless true. Unfortunately, after menopause, the ravages of collagenase go on due to estrogen-initiated production from adipose tissues. Sun damage and smoking are two other major causes of damaged skin, and although the skin can repair a lot of insults, these are beyond any that the body can reasonably repair. Following are ways to treat aging skin.
Start by advising clients to consume a good diet; avoid sugars and sweets, eat lots of fruits and vegetables, choose lean meats and fish, and avoid fried, broiled and roasted foods. Tell them to take a multivitamin daily, along with 100 mg of proanthocyanidins (OPC) and 500 mg of vitamin C daily.
Topical treatment should center on individualization. Basically, there are three degrees of damaged skin and remember, aging skin is damaged skin.
Minimal damage. Minimal damage results in fine lines around the eyes and mouth without sagging cheeks, and wrinkles that are a hair line in depth.
Topical treatment for minimal damage. Start with a chemical peel of either salicylic acid or lactic acid. Use a night cream with 0.1–0.5% retinol and vitamin C at 3–5%, and a collagenase inhibitor, such as OPC. During the day, use 5–10% vitamin C in a serum or cream, along with vitamin E and OPC, as well as an SPF of least 15. Remember that vitamin C will slow down sun damage. See the client every month for three months, then every two months. Lastly, if she smokes, it is crucial to try to convert her.
Moderate damage. Moderate damage appears as deeper, longer, stringlike lines around the eyes, with more pronounced nasolabial folds and the accentuation of the labiomental fold, as well as pigmentation changes as colored spots here and there, sagging cheeks and forehead wrinkles.
Topical treatment for moderate damage. Stop the damage and then reverse it. This will take some time, and perhaps a life change. Start with a salicylic acid peel and the day and night regimen as dictated for minimal damage treatment in the previous paragraphs. For the next visit in one month, do a Jessner’s peel, and schedule another appointment for the next month. The client will also benefit from an occasional oxygen treatment and an enzyme treatment. You can repeat the Jessner’s in three to six month and should recommend a vitamin C serum for daily use. Remember that vitamin C is essential in rebuilding collagen.
Severe damage. Severe damage is illustrated by very deep and long wrinkles everywhere on the face, much sagging, discoloration of all types and pigmentation disorders, eyes beginning to sink, deep folds of nasolabial and labiomental wrinkles, and a turkey neck.
Topical treatment for severe damage. Severely damaged skin is a real challenge. If the wrinkles are deep enough to hold a straw, you should refer a deep peel by a physician and follow up after the procedure. If this isn’t done, the client will need periodic Jessner’s peels, which should be preceded by an enzyme peel. Follow closely because the client may need retinol treatment levels from 1–2%. You should have experience and some training in these levels to use them. How often you perform a peel will depend on the client’s response and your level of skill.
Hyperpigmentation
There are three main types of skin hyperpigmentation.
- Melasma is a general term describing darkening of the skin.
- Chloasma is typically used to describe skin discolorations caused by hormones, such as pregnancy, birth control pills or estrogen replacement therapy. Frequently, melasma and chloasma are used interchangeably.
- Solar lentigenes is the technical term for darkened spots on the skin caused by the sun. Lentigenese describes a darkened area of skin that is quite common in adults with a long history of unprotected sun exposure.
Vitamin C can block tyrosinase, which is critical in the formation of melanin. It may take as long as 4–12 weeks to reduce pigmented spots. Vitamin C works well with lactic acid as a combined treatment. Magnesium ascorbyl phosphate, L-ascorbic acid, ascorbyl glucosamine and ascorbic acid are various forms of vitamin C considered stable and effective antioxidants for skin. It takes high levels of ascorbic acid to reduce pigment, up to 10% of some forms. Generally, ascorbic acid works best with other agents. Dermal pigment is diagnosed with a Wood’s lamp. A new observation indicates that a Wood’s lamp can be used to determine the depth of melanin pigmentation in the skin.8 Combining mandelic acid at 5% with ascorbic acid at 5–10% is often effective for dermal pigmentation. It is always best to refer a client with dermal pigmentation to a dermatologist. Your Wood’s lamp is one of the best diagnostic tools you have, so learn to use it well.
Scars and scar treatment
Facial scars are not easy to treat. Scar formation is a result of the process of wound-healing that promotes collagen formation. Vitamin C is essential in this process, but, at times, it will overproduce collagen, resulting in scar formation.
Hypertrophic scars are red raised lumps on the skin that are confined to the boundaries of the original wound. Although some are permanent, they can almost disappear after a few years.9
Keloid scars are large scars that constantly grow and often form into large, tumorous, benign growths and are most common in dark-skinned people. They can be caused by surgery; injuries; skin issues, such as acne; or body piercings, particularly on the ear lobes. A favorite site for keloids is the shoulders and the chest.9
Pitted scars are sunken recesses in the skin caused when underlying structures supporting the skin, such as fat or muscle, are lost, and are most commonly associated with acne and chicken pox.9
The average age of onset for keloids has been estimated to be approximately 22 years for both women and men. Specific body areas that are more prone to scars include areas with sebaceous glands that secrete oil or areas of high skin tension. They include the shoulders, chest, neck, upper back, ears and upper arms.10
Stretch marks, or striae, are also scars that can take many forms. They occur more frequently in females during adolescence and weight gain.
Some treatments for scars were developed a few years ago using silicone tapes. What these treatments seemed to do was to hydrate the scar, partially dissolving the collagen and stimulating the formation of new collagen. This is surely a simple explanation for what is a very complex process. Although they improved the scar, the skin was never returned to its original state.
Following are some suggestions for acne scars, surgical scars and burn scars. Remember, the treatments will take many weeks to many months to help, depending on the size and age of the scar. Use a vitamin C preparation with an occlusive dressing, such as plastic wrap or petroleum jelly. You will need a 3–5% concentration of ascorbic acid. If you notice irritation, change from crystalline ascorbic acid to magnesium ascorbyl phosphate. As you gain experience, you can increase the concentration of ascorbic acid, although it should not exceed 10%. Always do a small area first to gain experience with the method.
Derive the maximum benefit
Vitamin C is one of the most effective and important ingredients available to the esthetician to treat a number of skin problems, including aging skin, acne and pigmentation disorders. It is essential to understand the basic chemistry and physiology of vitamin C in order to derive the maximum benefit from its use. With experience, you will find it to be applicable to many of your clients’ most troubling skin care problems. Always keep in mind that vitamin C is a powerful treatment agent and does require both knowledge and experience to use it effectively and safely.
In the next part of this article, two fat-soluble vitamins that are very exciting agents for effective treatments will be covered. Alpha tocopherol, or vitamin E, is not just an antioxidant; you will discover other uses in skin care for this essential compound because it is absolutely critical for healthy skin. A new fresh look at vitamin D, or calciferol, shall be taken since it is a vitamin that has been almost totally neglected in skin care. Although one of the main uses for vitamin D is in the regulation of calcium, you will find many others for this versatile vitamin as it is pulled out of the shadows of neglect into the light of discovery.
REFERENCES
1. S Murad et al, Regulation of collagen synthesis by ascorbic acid, Proc Nat Acad Sci, 78 2879–2892 (1981)
2. M Kaminski and R Boal, An effect of ascorbic acid on delayed-onset muscle soreness, Pain, 50 317–321 (1992)
3. PJ Nelson et al, Effects of ascorbic acid deficiency on the in vivo synthesis of carnitine, Biochem Biophys Acta, 672 123–127 (1981)
4. AJ Brown, Acute effects of smoking cessation on antioxidant status, Nutr Biochem, 7 2939 (1996)
5. JR Hunt et al, Effects of ascorbic acid on apparent iron absorption by women with low iron stores, Am J Clin Nutr, 59 1381–1385 (1994)
6. S Wha Kim et al, Fibroblasts and ascorbate regulate epidermalization in reconstructed human epidermis, J Dermatol Sci, 30(3) 215–223 (Dec 2002)
7. S Pasonen-Seppänen et al, Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier, Histochem Cell Biol, 116(4) 287–297 (Oct 2001)
8. B Gilchrest et al, Localization of melanin pigmentation in the skin with Wood’s lamp, Brit J Dermat, 96 245–247 (2006)
9. en.wikipedia.org/wiki/Scar (Accessed Apr 14, 2009)
10. www.dowcorning.com/content/publishedlit/poster064.pdf (Accessed Apr 14, 2009)
Footnotes
a. Ascorbic acid means anti-scurvy agent. This term appears in 1937 and was devised by Albert von Szent-Gyorgyi Nagyrapolt in Hungary. He received the Nobel Prize in Physology or Medicine in 1937 for his isolation of ascorbic acid.
b. Malignant neoplasia is the medical term for cancer.
d. These markers are keratin 10, involucrin and filaggrin.
e. The word letigenes comes from the Latin word for lentil, a bean frequently used in soup.